EXTRACTS OF POMEGRANATE, PERSIMMON, NETTLE, DILL, KALE ANDSIDERITIS SPECIFICALLY MODULATE GUT MICROBIOTA AND LOCALCYTOKINES PRODUCTION: IN VIVO STUDY
- ediensofficial
- 27 жовт. 2025 р.
- Читати 15 хв

Підписуйтесь на наші соціальні мережі, щоб стежити за останніми новинами тут 💜:
Сайт: www.ediens.me
LinkedIn: www.linkedin.com/ediens
Instagram: www.instagram.com/ediens_official
TikTok: www.tiktok.com/@ediens_official
UDC 579.61
DOI: 10.15587/2519-8025.2020.204781
T. Meleshko, O. Pallah, V. Petrov, N. Boyko
Метою роботи було виявлення (in vivo, на моделі імунокомпетентних мишей) динаміки впливів перорального введених екстрактів їстівних рослин – інградієнтів традиційних хар- чових продуктів – на стан кишкової мікробіоти та продукування цитокінів слизовими обо- лонками кишечнику. Матеріали і методи. У дослідженні було сформовано сім груп імунокомпетентних мишей BALB/c, які протягом 14 днів перорально випоювались рослинними екстрактами (15 мг/200 мкл/миша). Останні одержували за розробленою нами технологією із харчових продуктів рос- линного походження – капусти кале, кропу, Sideritis scardica, кропиви, плодів хурми і гранату. Матеріалом мікробіологічного аналізу змін кишкової мікробіоти слугував вміст товстої кишки. Ключових представників мікробіоти ізолювали шляхом висіву серійних розведень на селективні хромогенні поживні середовища та ідентифікували серологічно і біохімічно. Продукцію цитокі- нів різними відділами кишечнику та лімфатичної тканини, асоційованої із слизовими оболонками кишечнику, визначали методом імуноферментного аналізу. Результати. В експериментах на мишах доведено здатність екстрактів капусти кале, кропу і Sideritis при їх оральному застосуванні вибірково інгібувати вміст E. сoli, K. pneumoniae, E. faecalis, L. acidophilus у товстій кишці мишей, стимулюючи при цьому ріст B. bifidum. Показано, що екстракт кропиви призводить до збільшення кількості E. coli, а екстракт хурми – до зрос- тання популяційних рівнів E. faecalis, Bifidobacterium та зменшення вмісту Candida spp. Екст- ракт гранату специфічно стимулює ріст Bifidobacterium. Існують значні відмінності у продуку- ванні цитокінів в супернатантах культури фрагментів тканин і в сироватці крові мишей при оральному введенні різних рослинних екстрактів. Екстракт кропу зумовлює системне і локальне збільшення ФНП-a та IЛ-2, а екстракти кропиви та Sideritis викликають таке збільшення лише на ділянках слизової оболонки. Екстракти гранату, хурми та капусти кале системно і локально стимулюють продукування ІЛ-2, ІЛ-10, ІЛ-12, ІФН-γ та ІЛ-17, але не ФНП-α. Висновки. Найбільш виразними корисними властивостями щодо впливу на стан кишкової мік- робіоти та продукування цитокінів слизовими оболонками кишечнику володіють екстракт хур- ми, а також екстракти гранату та капусти кале. Екстракти кропиви та кропу демонструють протизапальну дію. Екстракт Sideritis був нейтральним до більшості досліджуваних показни- ків. Жодний з екстрактів не виявив шкідливих впливів Ключові слова: екстракти їстівних рослин, імуномоделююча дія, кишкова мікробіота Copyright © 2020, T. Meleshko, O. Pallah, V. Petrov, N. Boyko. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0).
1. Introduction Human microbiome as well as how microorgan- isms affect the immune system is the frontier research topic. Host-microbial interaction and the possibility of targeted regulation of [gut] microbiota following to maintenance mucosal immune response is subject of great practical interest for prevention and treatment of noncommunicable diseases (NCD) initiated by low grade inflammation. The anti-aggregate effects of the same extracts of pomegranate, persimmon, nettle, dill, kale and Sideritis as well as their antioxidant properties by affect mole- cules related to endothelial NO release and vasodilation [1, 2]. However, their ability to modulate gut microbi- ome and local immune response was not studied (re- mains unclear). 2. Literature review Modern human nutrition needs to meet a lot of demands and provide not only safe and necessary com- position of micro and microelements, but also be func- tional, which means enable the prevention of the devel- opment or even treatment of various diet-associated dis- eases, such as type II diabetes (T2D), cardiovascular dis- eases (CVD), etc. [3]. The fruits, vegetables, whole grains and other tra- ditional foods are the major source of the biologically ac- tive components (BAC). It is largely known that bioac- tive compounds and their correlation with antioxidant capacity are insured of quality parameters typical/proved for the commercial fruit-based baby foods [4], and main- ly represented by vitamins, trace elements and antioxi- dants (polyphenols, anthocyanins, etc.) [5]. Scientific Journal «ScienceRise:Biological Science» No2(23)2020 5 It is known that these phytochemicals are influ- enced on the various important processes occurring in the human body, including gene expression, production of enzymes and hormones, activation of receptors, immune and antioxidant processes, etc. [6]. It is known that BAC have long been used in practical medicine, in particular, a well-known trend of the last decades is the construction of modern preparations based on them – prebiotics and synbiotics [7]. It is well known that the gut microbiota plays a major role in host health by shaping the development of the immune system, metabolizing dietary nutrients (such as fatty acids, glucose and bile acids) and drugs, digest- ing complex indigestible polysaccharides and synthesiz- ing vitamins and bioactive molecules [8, 9]. The changes of the gut microbiota may partially contribute to the higher incidences of chronic inflammatory disorders, such as cardiovascular disease, obesity, depression, aller- gies, diabetes and autoimmune disorders [9]. It has been shown that the gut microbiota is criti- cal for intestinal immune maturation [10]. Gut microbio- ta-stimulated immune maturation maintains gut homeo- stasis by protecting the host from infections [11], and damaging inflammatory responses [12]. It is therefore clear that it is essential to under- stand the biological interactions between the food ingre- dients, microbiota, and immune system. 3. The aim and objectives of the research The aim of this study was to use the in vivo model to reveal main changes in gut microbiota in dynamic of immunocompetent BALB/c mice and find out the speci- ficity of action of targeted foods/plants extracts on cyto- kines production in all gut compartments and GALT to its oral administration. To achieve the aim, the following tasks were set: 1. To detect changes in murine microbiota com- position as major players of regulation of mucosal im- mune response. 2. To measure production of pro-inflammatory and anti-inflammatory cytokines in fragment culture of small intestine and gut associated lymphoid tissues (GALT), including Payer Patches (PPs), peritoneal cavity (PC), mesenteries lymph nodes (MLN), following oral administration of selected edible plants extracts. 4. Materials and methods Plants/extracts/foods In our experiment we use extracts from six edi- ble plants obtained by our colleagues as part of the in- ternational project "BaSeFood" at the Institute of Food Research (Norwich, UK). Plants such as kale leaves (Brassica oleracea var. acephala), persimmon (Di- ospyros species), pomegranate (Punica granatum), dill (Anethum graveolens), Sideritis scardica, and nettle (Urtica dioica) were components of traditional food and were sourced from areas of the Black Sea region. Extraction of plant raw materials was carried out by the method of quantitative extraction by methanol ac- cording to the analytical scale. Fresh plant materials were weighed and introduced into the hot aqueous so- lution of methanol (65–70 °C) at a plant materi- al/aqueous methanol ratio of 1:10. Methanol-free ex- tracts of selected plants were obtained by vacuum evaporation. Animal groups In this study, seven groups of immunocompetent BALB/c mice were formed. All experimental mice had been fed orally by plants’ extracts (15mg/200 μl/mouse) for the 14th days. During the experiment, all animals were orally consuming different ingredient(s), in addition to the standard food received by the control group, de- pending on the experimental group: Group 1 – kale leaves extract; Group 2 – persimmon extract; Group 3– pomegranate extract; Group 4 – dill extract; Group 5 – Sideritis extract; Group 6 – nettle extract; Group 7 – control group, standard vivarium diet food. 3 animals were used per extract per 1 time point day 3rd, 7th, 14th, 21st, 28th and 1 month – wash out pe- riod – and were sacrificed and worked up separately. To determine local secretion of cytokines, tissue fragments (3 mm2 each) of the spleen, PPs, MLNs and small intes- tine: Duodenum (D), Jejunum (J), and Ileum (I) were cul- tured for 2 weeks in Kennet's HY medium in a microan- aerobic chamber at a ratio of CO2 and O2 gases of 90 % and 10 % respectively [13]. All experiments in mice were performed in ac- cordance with the international principles outlined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986) signed by the Verkhovna Rada of Ukraine in 2002, Law of Ukraine No. 3447 – IV “On the Protection of Animals from Cruelty”, meeting minutes of the Bioethics Commission of the Medical Faculty of the State University “Uzhhorod National Uni- versity” (Minutes No. 4, dated March 16, 2011). Microbiological assay In order to detect changes in murine microbiota composition on the 3rd, 7th, 14th, 21st, 28th days and 1 month from the beginning of the experiment 1 g of feces was collected from the experimental animals and mixed with 1 ml of PBS. Ten-fold serial dilution of samples was performed and plated correspondingly on the growth me- dia such as: RAPID’E.coli 2TM medium, UriSelect 4 (Bio-Rad, USA), Sabouraud Dextrose Agar, Lactobacil- lus MRS Agar, Bifidobacterium Agar, Bile Esculin Agar, MacConkey Agar (HiMedia Laboratories, India). Count- ing of all types of microorganisms was carried out ac- cording to the formula: CFU/g = a×b×c, where: a stands for the number of colonies grown on the nutrient medi- um; b stands for dilution coefficient dose (when plating 100 μl, a = 10; when seeding 50 μl, a = 5; when plating 10 μl, a = 100); and c stands for dilution factor. For bio- chemical identification semi-automatic systems (API BIOMERIEUX/LACHEMA/PAST tests, OKI diagnos- tics) had been used. ELISA The production of cytokines were detected by En- zyme-linked immunosorbent assay (ELISA) using the BioLegend’s ELISA MAXTM Deluxe Set Mouse IL-2, IL-10, IL-12, IL-17, TNF-a, IFN-g kitsand Immunoen- zyme Analyzer BioTek Elx800 at 450 nm. Serum and fragment culture samples were previously diluted in con- Scientific Journal «ScienceRise:Biological Science» No2(23)2020 6 centration 1:1, 1:5, 1;25 and 1:125, and all the samples (each mouse was worked out separately, each tissue: du- odenum, jejunum, ileum, PPs, MLNs were cultivated in triplicate for each time points: 3rd, 7th, 14th, 21st and 28th day and for the wash out period) were also meas- ured in triplicate. Statistics Statistical analyses were performed using the statis- tical program GraphPad Prism version 3.00. Tukey ́s and Dunnett ́s tests (after analysis of variance, ANOVA) were used to identify the differences between groups. In tables are presented results from Dunnett ́s test –meaning that all experimental groups were compared against control. 5. Research results and discussion Changes in mice (BALB/c) gut microbiota tested in gut content The short-term effects of extracts on the composi- tion of the gut microbiota were studied. Gut content was collected: 1) immediately after oral administration of select- ed plants’ extracts (0 or 1 hour), 2) 24 hours, and 3) 48 or 72 hours. The Fig. 1 is presenting the overall (panoramic) results of this first preliminary acute experiment, namely the effect of each of the tested extracts in the first 48– 72 hours of mice feeding on the colon microbiota. In the second performed experiment the long-term effects of the same extracts on the gut microbiota of BALB/c mice has been studied. Persistence of gut mi- croorganisms had been detected in colon in another ex- periment when mice were sacrificed on days 3rd, 7th, 14th, 21st, and 28th wash out period (Fig. 2–8 including the control).





On the mouse model it was shown that the extract of dill inhibited two Enterococcus strains (E. faecalis and E. faecium), Klebsiella pneumoniae and Lactobacillus spp., and stimulated Bifidobacterium bifidum. Bifidobac- teria were also stimulated by the kale extract, but in this case there were no significant effects on any of the other tested gut microbiota. The nettle extract caused a non-specific stimula- tion of gut microbial composition on day 3, whereas by days 14 and 24 all the indices were approximately equal to their initial levels (for E. coli and K. pneumoniae), but lactobacilli and bifidobacteria were both dramatically re- duced. Persimmon extract was the only extract able to specifically stimulate lactobacilli, while the pomegran- ates extract specifically stimulated bifidobacteria. The Sideritis extract effectively inhibited K. pneu- moniae and commensal E. coli, and also induced a statisti- cally significant increase in bifidobacteria and a moderate but non-significant increase in lactobacilli (Fig. 9). Effect of edible plant extracts on cytokines pro- duction The pro- and anti-inflammatory cytokine profiles in all specimens of isolated tissue fragments (parts of the small intestine – duodenum, jejunum and ileum, MLN, PPs) and serum of the BALB/c mice after oral admin- istration of the plants’ extracts had been determined. The differences in the levels of cytokines pro- duced in the culture supernatants of tissue fragments and in the serum of mice with the oral administration of vari- ous plant extracts has been detected. In Fig. 10–12, the data obtained for kale, persimmon and pomegranate ex- tracts on days 3, 7, 14, 21 and 28 of the experiment is in- troduced. Consumption of dill extract has the ability to stimulate the production of only IL-2 in the range from 2.35 pg/ml in the duodenum to 2 pg/ml in serum. Similar is the effect of Sideritis extract consumption, which also stimulates to a large extent the synthesis of IL-2 locally, in the duodenum, jejunum and ileum from 2.11 pg/ml to 2 pg/ml had been shown.

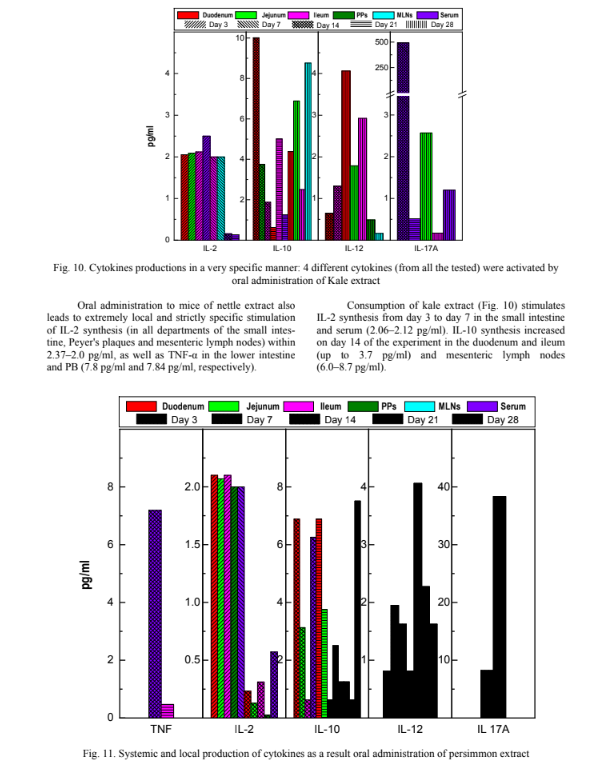
Unlike IL-10, interleukin 12 is most actively pro- duced on the 21st day of the experiment to 4.63 pg/ml). It should be noted that on the 14th and 21st day of the ex- periment there was an increase in the amount of IL-17A to 493.5 pg/ml in serum of laboratory animals, and on the 28th day – in the MLNs (2.5 pg/ml), which is evidence of its proinflammatory action. We found that persimmon extract (Fig. 11) has a distinct systemic and local proinflammatory effect throughout its oral administration (ie, within 14 days). In particular, there was a sharp increase in TNF-α in serum of laboratory animals on the 14th day of the experiment (up to 7.1 pg/ml), on the 7th and 14th day, the synthesis of IL-17A in the serum of animals increased (8.22– 38,34 pg/ml), the synthesis of IL-2 (up to the level of 2.1–2.7 pg/ml) also increases on the 3rd and 7th days of oral administration of this extract. From the 14th to the 21st day of the experiment the synthesis of IL-10 (up to 6.8–6.85 pg/ml) is increased, with a further decrease on the 28th day (2.4–1.3 pg/ml) and vice versa, the level of its IL-12 agonist increases significantly, starting from day 14 of the experiment in all departments of the small intestine (4.06 pg/ml), MLN (2.27 pg/ml) and in serum (1.8 pg/ml).

Thus, the persimmon extract induces a local pro- inflammatory effect in the initial terms after oral admin- istration. Oral administration to mice of pomegranate ex- tract (Fig. 12), similar to persimmon extract, stimulates the synthesis of IL-2 locally on the 3rd and 7th day (up to 2.11 pg/ml) with a tendency to its sharp decrease on the 14th, 21st and the 28th day of the experiment (up to 0.7– 0.5 pg/ml), and on the 21st day is characterized by a clear pro-inflammatory effect (IFN-γ synthesis reaches the level of 47.81 pg/ml, IL-17A–500 pg/ml). The optimal effect of this extract is observed on the 14th day of its oral administration, when the levels of proinflammatory cytokines become lowest and, conversely, the levels of anti-inflammatory cytokines (IL-12) reach a maximum in the departments of the small intestine, MDL and PB (up to 2.4–4.38 pg/ml). 6. Discussion In our study we investigated the ability of ex- tracts of pomegranate, persimmon, nettle, dill, kale and Sideritis to modulate gut microbiome and local produc- tion of cytokines. The results of the study show no harmful influence of tested plants on murine gut mi- crobial composition still based on our investigation we can conclude that most beneficial properties are inher- ent to persimmon and then pomegranate and kale. Si- derites rather show no significant influence on all the studied indices while the Nettle and Dill are acting pro- inflammatory. Analysing the obtained experimental data we can note that secreting of targeted cytokines initiated by oral administration of investigated plants’ extracts is very specific and depends on the time point of exper- iment and its locations (SI, PPs, MLNs vs. serum). Scientific Journal «ScienceRise:Biological Science» No2(23)2020 13 Obtained experimental data indicate the advisa- bility of using extracts as natural strictly specific reme- dy of prevention and treatment of diet-associated dis- eases by balancing of gut microbiota and immune sys- tem. It should be noted that experimental works on the study of the specificity of action of targeted foods/plants extracts on gut microbiota and local im- mune response is not so much and mostly these re- searches are directed on studying of influence of each extracted component separately. Zhu et al. showed that supplementation with low dose of persimmon tannin induced a significant alteration in specific bacterial species by increasing some types of gut microbiota that have been inversely related to obesity (Bifidobacterium spp., Lactobacillus spp.) and reducing the relative abundance of others associated to diet-induced obesity (E. coli and Enterococcus) [14]. However, analysing the experimental data we obtained, the Persimmon ex- tract decreased numbers of lactobacilli on 7th day of oral administration but after the wash-out period was no statistical differences observed. Interestingly, com- mensal E. coli and Enterococcus was not affected by Persimmon extract. George et al. also documented that a pomegranate peel extract treated decreased levels of Lactobacillacieae in mice gut microbiota [15]. Howev- er, our obtained experimental data showed that lacto- bacilli were stimulated by Pomegranate on 3 day of oral administration but after wash-out period no statis- tical differences was observed. In our study we did not make detection of amount of total/activated B1/B2, CD4/CD25, CD8, CD56 other immune cells subsets in spleen and differ- ent components of GALT. Further research can be related with the using of in vivo model to reveal the specificity of action of selected foods/plants extracts on gut microbiota and immune re- sponse of mice with combined T-and B cells deficiency. 7. Conclusions 1. Number of Lactobacilli strains in gut content was not significantly affected by Dill and Sideritis ex- tracts; and were stimulated by Pomegranate extract on 3, inhibited by Kale extract on 3–14 d and by Persimmon extract on 7 d; after the wash-out period no statistical dif- ferences was observed. K. pneumoniae and E. cloacae were eliminated from mice colon by feeding of the ex- tracts of Sideritis, dill, nettle and persimmon but not pomegranate and kale. E. faecium and E. faecalis was stimulated significantly by pomegranate extract. B. bi- fidum and B. longum were increased only in colon content of mice fed with extracts of Sideritis, pome- granate and kale. Interestingly, commensal E. coli was not affected by any of the tested plants’ extracts sig- nificantly. All plants’ extracts had promoted C. albi- cans persistence in mice colon; only kale extract had lowered its amount on 7–14 d but not on 21–28 d and after the wash-out period. 2. There are sufficient differences in produced cytokines in FC and serum of mice fed with different plants extracts. TNF-a, and IL-2 increased both system- ically and locally in the different gut compartments by dill, nettle and Sideritis extracts only at mucosal sites. IL-2, but also IL-10 and IL-12, IFN-g, and IL-17 but not TNF-a were stimulated in different levels by pome- granate, persimmon and kale extracts: both systemically and locally. Conflicts of interest The authors declare that they have no conflicts of interest.
References 1. Woodcock, M. E., Hollands, W. J., Konic-Ristic, A., Glibetic, M., Boyko, N., Koçaoglu, B., Kroon, P. A. (2013). Bioac- tive-rich extracts of persimmon, but not nettle,Sideritis, dill or kale, increase eNOS activation and NO bioavailability and decrease endothelin-1 secretion by human vascular endothelial cells. Journal of the Science of Food and Agriculture, 93 (14), 3574–3580. doi: http://doi.org/10.1002/jsfa.6251 2. Konić-Ristić, A., Srdić-Rajić, T., Kardum, N., Aleksić-Veličković, V., Kroon, P. A., Hollands, W. J. et. al. (2013). Effects of bioactive-rich extracts of pomegranate, persimmon, nettle, dill, kale andSideritisand isolated bioactives on arachidonic acid in- duced markers of platelet activation and aggregation. Journal of the Science of Food and Agriculture, 93 (14), 3581–3587. doi: http://doi.org/10.1002/jsfa.6328 3. Pallah, O. V., Meleshko, T. V., Bati, V. V., Boyko, N. V. (2019). Extracts of edible plants as beneficial microorganisms growth stimulators. Biotechnologia Acta, 12 (3), 67–74. doi: http://doi.org/10.15407/biotech12.03.067 4. Carbonell-Capella, J. M., Barba, F. J., Esteve, M. J., Frígola, A. (2013). Quality parameters, bioactive compounds and their correlation with antioxidant capacity of commercial fruit-based baby foods. Food Science and Technology International, 20 (7), 479– 487. doi: http://doi.org/10.1177/1082013213492523 5. Moyer, R., Hummer, K., Wrolstad, R. E., Finn, C. (2002). Antioxidant compounds in diverse ribes and rubus germplasm. Acta Horticulturae, 585, 501–505. doi: http://doi.org/10.17660/actahortic.2002.585.80 6. Correia, R. T., Borges, K. C., Medeiros, M. F., Genovese, M. I. (2012). Bioactive compounds and phenolic-linked func- tionality of powdered tropical fruit residues. Food Science and Technology International, 18 (6), 539–547. doi: http://doi.org/10.1177/1082013211433077 7. Perez-Gregorio, R., Simal-Gandara, J. (2017). A Critical Review of Bioactive Food Components, and of their Functional Mechanisms, Biological Effects and Health Outcomes. Current Pharmaceutical Design, 23 (19), 2731–2741. doi: http://doi.org/10.2174/1381612823666170317122913 8. Lankelma, J. M., Nieuwdorp, M., de Vos, W. M., Wiersinga, W. J. (2015). The gut microbiota in internal medicine: impli- cations for health and disease. The Netherlands journal of medicine, 73 (2), 61–68. 9. Oriach, C. S., Robertson, R. C., Stanton, C., Cryan, J. F., Dinan, T. G. (2016). Food for thought: The role of nutrition in the microbiota-gut–brain axis. Clinical Nutrition Experimental, 6, 25–38. doi: http://doi.org/10.1016/j.yclnex.2016.01.003 10. Chung, H., Pamp, S. J., Hill, J. A., Surana, N. K., Edelman, S. M., Troy, E. B. et. al. (2012). Gut Immune Maturation De- pends on Colonization with a Host-Specific Microbiota. Cell, 149 (7), 1578–1593. doi: http://doi.org/10.1016/j.cell.2012.04.037 Scientific Journal «ScienceRise:Biological Science» No2(23)2020 14 11. Duan, J., Chung, H., Troy, E., Kasper, D. L. (2010). Microbial Colonization Drives Expansion of IL-1 Receptor 1- Expressing and IL-17-Producing γ/δ T Cells. Cell Host & Microbe, 7 (2), 140–150. doi: http://doi.org/10.1016/j.chom.2010.01.005 12. Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y. et. al. (2010). Induction of Colonic Regula- tory T Cells by Indigenous Clostridium Species. Science, 331 (6015), 337–341. doi: http://doi.org/10.1126/science.1198469 13. Manohar, M., Baumann, D. O., Bos, N. A., Cebra, J. J. (2001). Gut Colonization of Mice withactA-Negative Mutant of Listeria monocytogenesCan Stimulate a Humoral Mucosal Immune Response. Infection and Immunity, 69 (6), 3542–3549. doi: http://doi.org/10.1128/iai.69.6.3542-3549.2001 14. Zhu, W., Lin, K., Li, K., Deng, X., Li, C. (2018). Reshaped fecal gut microbiota composition by the intake of high mo- lecular weight persimmon tannin in normal and high-cholesterol diet-fed rats. Food & Function, 9 (1), 541–551. doi: http://doi.org/10.1039/c7fo00995j 15. George, N. S., Cheung, L., Luthria, D. L., Santin, M., Dawson, H. D., Bhagwat, A. A., Smith, A. D. (2019). Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food science & nutrition, 7 (8), 2565–2576. doi: http://doi.org/10.1002/fsn3.1106 Received date 12.02.2020 Accepted date 06.03.2020 Published date 30.04.2020 Tamara Meleshko, Senior Lecturer, Junior Researcher, Department of Clinical and Laboratory Diagnostics and Pharmacology, RDE Centre of Molecular Microbiology and Mucosal Immunology, State Higher Educational In- stitution «Uzhhorod National University», Narodna sq., 3, Uzhhorod, Ukraine, 88000 E-mail: meleshkotv@ukr.net Oleksandra Pallah, Assistant, Junior Researcher, Department of Clinical and Laboratory Diagnostics and Pharmacology, RDE Centre of Molecular Microbiology and Mucosal Immunology, State Higher Educational In- stitution «Uzhhorod National University», Narodna sq., 3, Uzhhorod, Ukraine, 88000 E-mail: ssarvash@gmail.com Viktor Petrov, PhD, Assistant, Department of Clinical Disciplines, State Higher Educational Institution «Uzhhorod National University», Narodna sq., 3, Uzhhorod, Ukraine, 88000 E-mail: petrovviktor.uzh@gmail.com Nadiya Boyko, Doctor of Biological Sciences, Professor, Head of Department, Director of Center, Department of Clinical and Laboratory Diagnostics and Pharmacology, RDE Centre of Molecular Microbiology and Mucosal Immunology, State Higher Educational Institution «Uzhhorod National University», Narodna sq., 3, Uzhhorod, Ukraine, 88000 E-mail: nadiya.boyko@gmail.com



Коментарі